From Proof of Concept to Production –
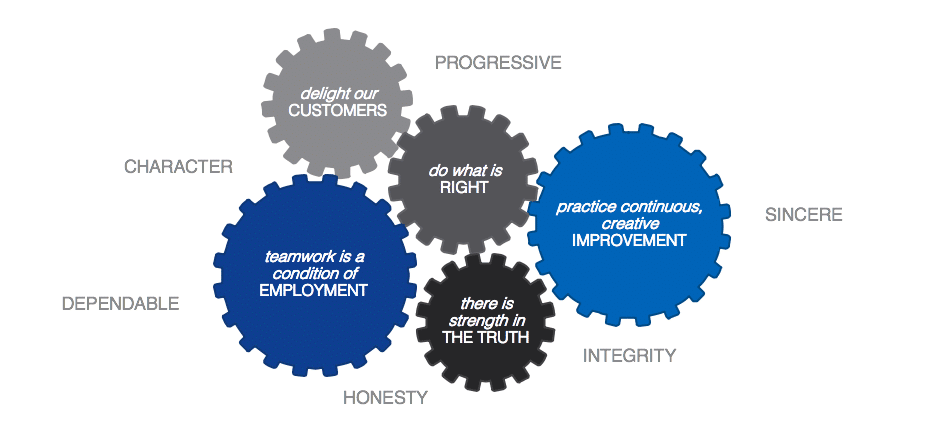
We Stand By Our Principles
Electronic Manufacturing Services and Systems Integration
SMC offers a complete solution for your EMS and Systems Integration needs. We have separate focused engineering groups for Customer Engineering, Test Engineering, Process Engineering, Quality Engineering, and Systems Integration, as well as a very strong Strategic Business Management role. SMC provides technical resources for New Product Introduction (NPI), through all levels of volume production.
We are customer-focused and reach out to companies that need the manufacturing services support we provide. Our commitment to Principles, People, Process, and Performance (the 4 P’s), gives us the confidence to offer direct fulfillment to the end customer. SMC believes in a genuine, integrated partnership. SMC is ISO 13485:2016 Registered for the Medical Device Industry, ISO 9001:2015 Registered and FDA Registered (Registration Number 3009627223) and manufactures to a Certified IPC-A-610 Rev G.
SMC’s operations team is made up of highly skilled engineers and professionals, most of whom have 30+ years of experience assembling high tech products.
TIER 1 OFFERINGS
Design Review
- Analysis for Manufacturability
- Analysis for Testability
- Analysis for Procurement
NPI
- Rapid Prototype
- Pre-Production Build
PCB Assembly
- L-M-H Volume
- Complex PCBA
- ICT and Functional Test
- R.F. Experience
Systems Integration
- Sub Assembly
- Complex Box Build
- Full Assembly Testing
- Custom Product Configuration
Logistics
- Returnable Packaging
- Customer Warehousing
- Direct fulfillment

